|
Do
you really use up all of the limiting reagent?
|
Now place 8 moles of N2 into the reaction chamber
and 5 moles of H2 and press "equilibrate". Obviously
the applet solves for the equilibrium concentration of each species. However,
let's say that you were going to solve the equilibrium problem by hand
instead of letting the applet do the math for you. On a separate sheet
of paper write down the equilibrium constant expression.
(Hint: K = products over reactants etc.)
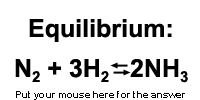
Also on a separate sheet of paper make the equilibrium table that has "initial concentration", "change" and "concentration at equilibrium".
