|
Concepts
and Problems to Work
|
- Do you really use up all of the limiting agent?
- How the magnitude of "K" affects
the extent of reaction.
- If we change the initial concentrations do the
equilibrium concentrations change?
- A
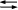 B K = 2 versus B
B K = 2 versus B 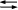 A K = ½
A K = ½
- Solving an equilibrium problem when both products
and reactants are present initially.
- Getting an equilibrium from "all products".